The Fat Primer
If you're afraid of butter, use cream.
-Julia Child (1912-2004)
The subject of fats is downright confusing, no doubt about it. My aim with the Fat Primer is to take some of the mystery out of this high energy food group (9 calories/gram, 28g/ounce!) by giving you a basic, easy to grasp look at the world of fat.
To understand fats, you need to be willing to endure a little bit of chemistry. Don't worry! I'll keep it simple and use lots of pictures. Many apologies in advance to my chemistry profs for leaving out so many details!
Let's start with the raw materials for all fats: carbon, hydrogen and oxygen. Certain atoms can hold on to, or 'bond' with other elements. Carbon, for instance, has 4 bonds available. Oxygen has two, hydrogen, only one.
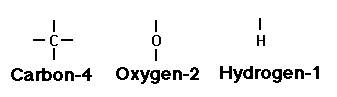
Seems simple enough, but what a dizzying array of things you can make with just these three simple elements! We'll stick with the fats for now, though, and consider one last thing about carbon. Two carbon atoms next to each other can share either one or two bonds in the fats you're likely to find in raw milk:
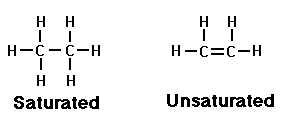
The type of bond determines the number of hydrogens each carbon can hold. The two carbons to the left, above, held together by a single bond, are said to be 'saturated' because they're holding all the hydrogens they can. That's all saturated means. Nothing to do with being wet!
The two carbons above on the right, connected by a double bond, are 'unsaturated' because each could possibly hold on to one more hydrogen if they weren't busy being so friendly with one another.
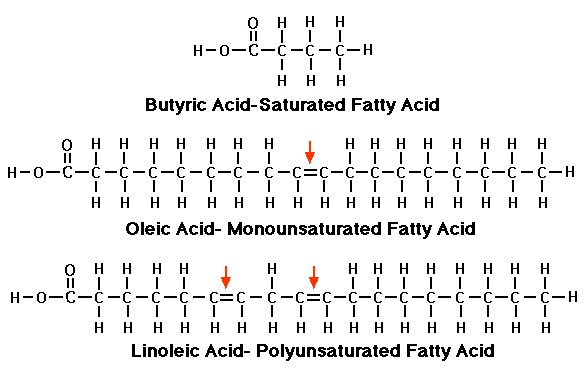
Because of the interactions of adjacent atoms, fatty acids with only single bonds tend to be straight and more stable than those with double bonds.
Molecules tend to kink where there's a double bond- the main reason saturated fats like butter and lard are solids at room temperature (the straight chains fit together tightly), and vegetable oils with their multiple double bonds are liquids (the kinks keep them from packing as closely)- think of uncooked, parallel spaghetti vs. elbow macaroni, for instance.
The building blocks of all fats are called fatty acids. Simply put, they're chains of carbon atoms connected like beads on a string, with hydrogen atoms on each carbon and an acid (carboxyl) group on one end.
The number of carbon atoms in each fatty acid chain, the type of bonds between the carbons, and how many hydrogens the carbons are holding on to, all determine the type of fat and its characteristics. Fatty acids with one double bond in the chain are called monounsaturated, with two or more, polyunsaturated.
Most fats in our bodies and foods are in the form of 'triglyceride' molecules = one glycerol (or glycerin, a sugar alcohol) + three fatty acids attached:
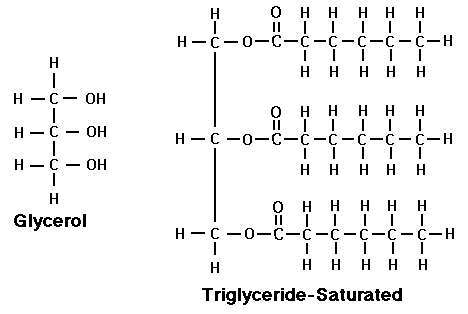
High levels of triglycerides in the blood have definitely been linked to the potential for heart disease. Their source is not from fats in the diet though, but rather from excess carbohydrates not burned for energy.
Overeating sugar or white flour products causes a spike in insulin, the hormone which, besides controlling blood sugar, also triggers the formation of fats destined for storage. The fats in raw milk aren't the problem. It's the half a bag of chocolate chip cookies you're dunking in it that's setting you up for circulatory grief...
To complicate things a bit further, and because chemistry wouldn't be chemistry if it didn't throw the occasional curveball, unsaturated fats can have different shapes or configurations depending on which side of the double bond the hydrogens end up.

This concept is important because the shape of the whole triglyceride molecule, and thus it's properties and characteristics, is affected by what happens at each double bond.
As you can see, in a 'cis' (pronounced 'siss') formation, the hydrogens are on the same side. With 'trans' formations, they're on opposite sides of the double bond. The linear stick and letter figures I've been using don't really show the true shapes of the cis/trans unsaturated fatty acids accurately. In actuality, as mentioned above, there's a lot of bending going on whenever double bonds enter the scene.
Trans fatty acids have been in the news quite a bit recently, especially now that food manufacturers have to disclose their presence on package labels, but we have to backtrack a bit to see why.
When vegetable oil processors thought it would be cool to make their products stay solid at room temperature, like butter and lard, they came up with a process called hydrogenation which yielded margarine and shortening. Crisco, by the way, of which I must have eaten a ton in baked goods when I was a kid, is hydrogenated cottonseed or soybean oil.

Nobel Prize winner Paul Sabatier (1854-1941, at right) is considered the father of the hydrogenation process. He discovered in 1897 that the metal, nickel, catalyzes, or facilitates, the attachment of hydrogen to carbon compounds.
In the actual process, workers heat the oil to very high temperatures and bubble hydrogen gas through it in the presence of nickel or some other catalytic metal. Since the vegetable oils are unsaturated, they can take on a few more hydrogens.
When they do, the molecule stiffens, and the fat is now closer to a solid. They can control just how firm it gets by how long they pump the gas through. That's why you'll sometimes see the term 'partially hydrogenated' on ingredient labels.
What also happens during hydrogenation, or later, during high heat cooking with the processed oils, is the formation of molecules so strangely configured that they're completely unsuitable for use in our bodies.
As an added bonus, the double bonds in these foreign fatty acids are easily broken, allowing the formation of free radicals- highly reactive molecules with an unpaired electron, just looking for something to grab on to.
Promotion of breast cancer, heart disease, diabetes, weakened immune systems and hormonal dysfunction are just some of the maladies for which studies have implicated these unnatural trans fats.
The point I'm trying to make in presenting all this information is that, yes, there are bad fats, but there are good fats, as well. Consider that the traditional fats eaten by our ancestors, and cultures around the world, were more often saturated than not, but that cardiovascular disease was almost unknown before the introduction of hydrogenated vegetable oils.
Our bodies badly need saturated fats- they make up half or more of our cell walls, they bolster our immune systems, nourish our heart muscle, carry important fat soluble vitamins and antioxidants, and, in the case of butter, contain anti-fungal, anti-microbial and anti-cancer agents.
Here's the good news: the saturated fats in clean, raw milk can do all of that for you, and much, much more.