Calcium

Raw milk is an excellent source of calcium, not only due to the high amount it contains, but also because of its bioavailability. The processing most modern dairy products undergo today impacts just how much of the calcium is available for the body to use, and, just as critical,
where it's used (and where it ends up). To understand calcium's importance in maintaining our health, let's look at some of its properties and functions.
When most people think of calcium, they picture something like the chalky cliffs of Dover (
above, right), made of calcium carbonate. Actually, calcium's a member of the alkaline metal family of elements, but you'll never find it in metallic or pure form in nature. That's pretty remarkable considering it's the 5th most abundant substance on Earth, and is testimony to its ability to combine with a broad range of substances.

Named after the Latin word calx for lime (calcium oxide, not the fruit!), calcium was used in ancient Rome as early as the 1st century. It wasn't until 1808, though, that it was isolated in a nearly pure metallic form by Sir Humphrey Davy (1778-1829, right), a brilliant scientist who also discovered/purified magnesium, strontium and barium.
It's all around us in everyday life- in cement as calcium oxide (CaO), plaster of Paris (calcium sulphate, CaSO4), limestone and sea shells (calcium carbonate, CaCO3)- and it's in us in a big way, teamed up with phosphorus as hydroxyapatite. Calcium is the most abundant element in our bodies, with 98% of it found in our bones and teeth, the rest distributed in ionic form throughout our system in blood, cells and tissues.
Since calcium plays such a crucial role in so many areas of our physiology, I'll just list the major ones here. Aside from the obvious contribution to teeth and bones, it figures heavily in muscle contraction, nerve impulse transmission, heartbeat regulation, blood clotting, enzyme activation, even fluid balance in our cells.
To get the most of it from our food, a number of conditions have to be just so. For instance, vitamin D is needed for proper calcium absorption. So is adequate amounts of the amino acid lysine. Also critical is the presence of phosphorus. A calcium to phosphorus ratio (Ca/P) of from 2:1 to 1:1 has been shown to promote the highest levels of absorption.
The ratio in raw milk is approximately 1.3:1 which falls nicely within the optimum range. When the dietary Ca/P ratio moves the other way, toward, say, 1:2, as it might with high-protein diets or heavy consumption of carbonated soft drinks, calcium loss is elevated.
Other factors in foods can block the absorption of calcium or cause its unwanted secretion as well. For instance, sodium and caffeine both elevate calcium levels in the urine. Every cuppa (equivalent to 150 mg of caffeine), can cause the loss of 5mg of calcium. Foods that contain oxalic and phytic acids, such as rhubarb, spinach and soybeans, can block the absorption of calcium and other key nutrients.
How much is enough every day? The graph below shows that needs vary with age, gender and other conditions, but there's little agreement on just what amount is appropriate. Some scientists abhor supplementation, others believe it's the only way to ensure adequate levels. In truth, there are so many variables relating to calcium and its use in people's bodies that studies often end up with conflicting results.
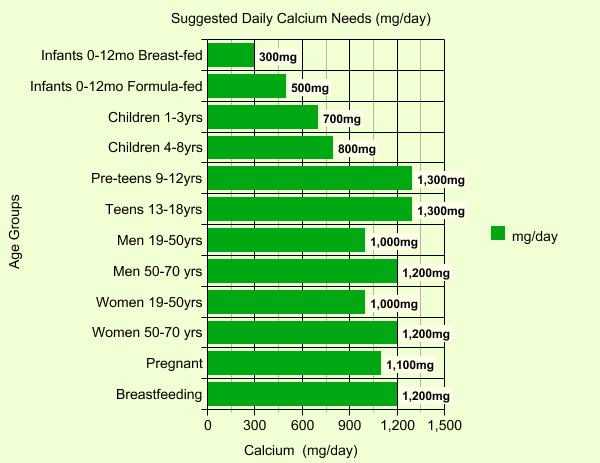
There is a consensus on one point however- most people aren't getting enough in their diets to maintain good health (usually only 500-800mg daily at most). Certainly, knowing that there are periods in your life when higher levels are justified is a start in the right direction toward making sure those needs are met.
Take female athletes and menopausal women- both groups need more dietary calcium because their estrogen levels are lower, and reduced estrogen levels can speed up mineral loss from bones.
Pregnant women benefit from higher levels for obvious reasons, but calcium is also effective in protecting mothers-to-be (especially those under 20 years old) from the development of pre-eclampsia, a high blood pressure condition that's one of the leading causes of pregnancy deaths. Adequate levels of calcium have also been shown to help ward off colon cancer.
It's plain to see calcium is essential to our health and well-being. Choosing raw milk and other unpasteurized dairy products (8 ounces of milk contains about 300mg) for a significant portion of your daily calcium needs makes good sense for a number of reasons: the calcium is more bioavailable than in heated dairy products, the calcium you get from raw dairy gets delivered where it's needed, not deposited in joints and arteries, and best of all, the taste is delicious. Make raw dairy, whether fresh or fermented, a significant portion of your daily diet and reap the benefits. Your bones will thank you!